In order to locate pre-symptomatic and asymptomatic patients quickly and massively, and in order to cut the chain of infections, a significant increase in the number of tests is needed.
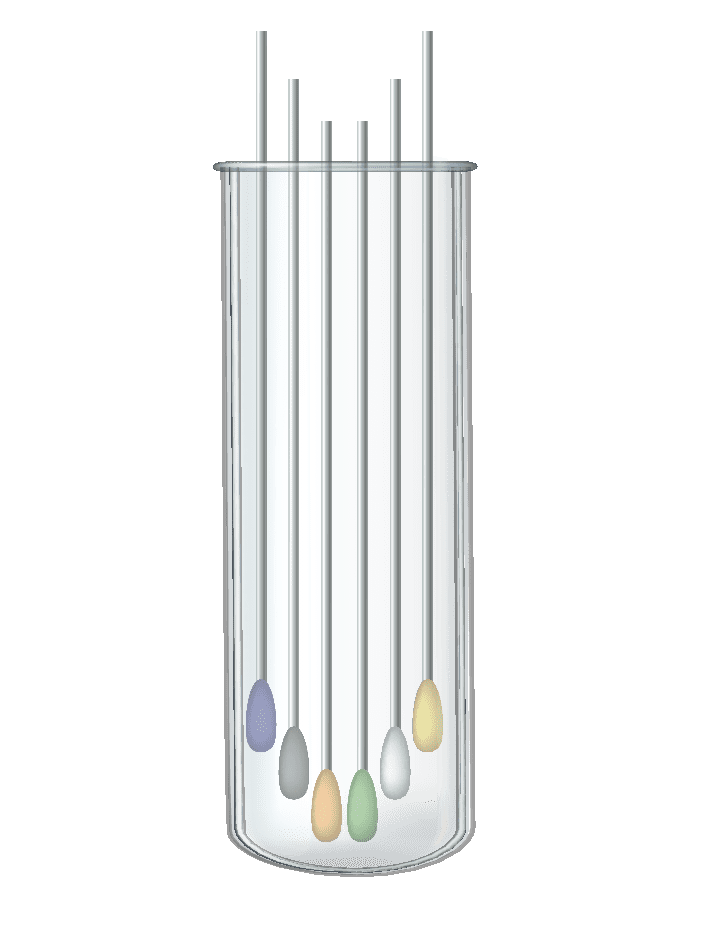
The optimization of the test set for capsules can be achieved by “swabs pooling” – a method by which many test subjects can be tested together without changing the test load in laboratories.
The principle is to use group sampling, instead of individual sampling.
Epidemiological models show that in order to cut the chain of infections, the scope of tests and the speed of response are more important than the sensitivity of the test.
The Method
What is Swab Pooling?
A group test, in which each subject is tested by a separate swab, and all the swabs are placed in the same test tube. The laboratory tests one test tube via the normal test process, which contains several samples, thus the workload does not change. If one of the subjects is positive – the whole group is instructed to quarantine, and personal tests are performed for each of the subjects.
Pros:
- No increase in lab workload
- A significant growth in the number of subjects tested
- No change in the number of test tubes
- Ease of Sampling: can be done by a medic / staff member / adult or self-sampling – either under supervision or after demonstration
Cons:
- The individual sensitivity of the test is limited
- It is not possible to know who is the verified patient in the group
Objectives
- Enable control of the infection and spread of the virus in Israel at a national strategic level, together with local control.
- Conduct extensive Sickness Surveys in specific locations where the disease is highly spread, in order to reduce morbidity and cut infection chains.
- Clinical trials.
- Critical facilities and essential factories, places with a very high infection potential, tests before and after special events.
- Testing to reduce the risk of infection due to travel between different areas: outline for opening the sky to aviation, tests for temporary release (24-48 hours) from isolation or lockdowns.
System applications
Potential subjects:
group that lives and works together and can be effectively quarantined.
Possible applications:
- Extensive population surveys to detect pre-symptomatic and asymptomatic.
- Nursing homes – periodic tests every 3 days for all staff members.
- Educational Institutions – testing all lower grades in four days, prior to school opening. Each grade will be pool tested – one test tube.
- Critical / high-risk populations.
- Neighborhood testing – testing all the households in a critical area, in one day. Each family will be pool tested (one test tube).
If a test is positive:
- The whole group goes into isolation.
- Sample all subjects individually.
- Epidemiological Investigation.
THE COMMON POOLING PROCESS
Swab pooling
Normal lab
procedure
SWAB POOLING ALTERNATIVES
Pooling dry swabs, in the lab – where another procedure is required.
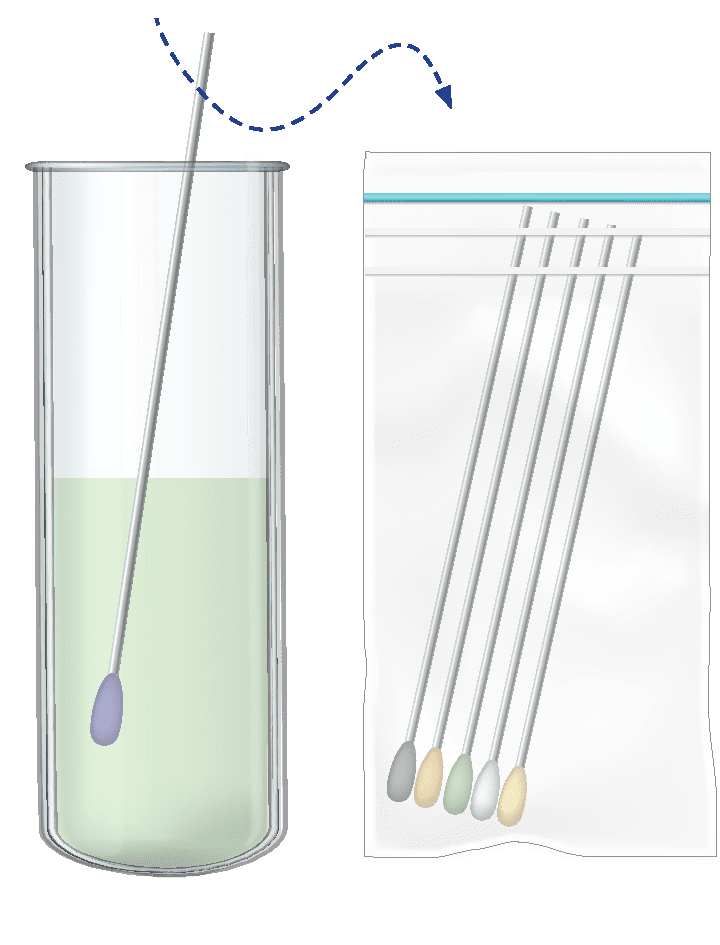
Dipping swabs and disposing them into a bio-hazard bag at home.
Then sending tubes to the lab with solution only.

Pooling swabs to lysis buffer
solution.
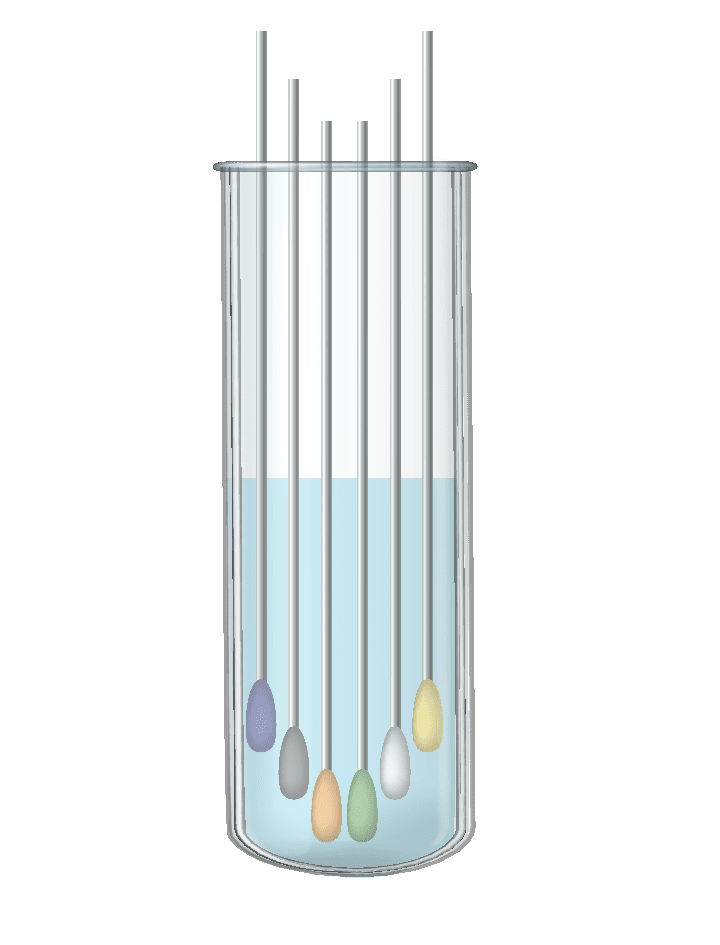
Pooling swabs into UTM.
Pilot
Performing the pilot on two groups:
- Pre-school teachers and assistants in Bnei Brak: 700 women (30/08).
- Yeshiva students, 1500 men (31/08).
Goals:
- Measuring reliability of tests.
- Examining the logistics operation – sampling, collecting test tubes, marking test tubes, laboratory, returning results reliably.
- Examining the system’s contribution to lowering the morbidity level.
- Examining value for money.
- Examination of the performance of the personal tests after the detection of a positive capsule.
Preparations:
- Large number of swabs.
- Preparation of test tubes with buffer lysis fluid.
- Transporting the samples to the subjects and to the laboratories.
- Evaluations for the system that reports the group test results.
- Establish a positive capsule treatment policy.
OPERATION
2133 participants
Two days
Pre-school teachers and assistants in Bnei Brak, yeshiva students in 4 different yeshivas
Divided into 5 types of groups with different number of participants.
Performed by Magen David Adom forces
2 samples per subject:
Swabs Pooling (muzzle, one nostril).
Standard personal test (muzzle, second nostril).
Testing the samples in two different laboratories.
Results were recorded separately
Individual sampling and group sampling for verification
Comparing the individual tests results to the group tests results
Process
- Writing a protocol together with the laboratories.
- Planning of capsules for testing.
- Logistics preparation.
- Registration of subjects.
- Capsule sampling in individual and group tests.
- Performing laboratory tests.
- Comparison of individual tests and group tests.
- Quarantine for positive capsules.
- Individual tests for positive capsules.
Number of participants
For full view change to horizontal
| Number of group tests | Number of pilot participants | Number of individual tests | Test group |
|---|---|---|---|
| 46 | 654 | 742 | Pre-school teachers, Bnei Brak |
| 20 | 587 | 682 | Hevron yeshiva, Jerusalem |
| 10 | 266 | 279 | Netiv HaDaat yeshiva, Jerusalem |
| 11 | 344 | 356 | Mishckenot HaTora yeshiva, Bnei Brak |
| 18 | 282 | 351 | Rina Shel Tora yeshiva, Karmiel |
| 106 | 2133 | 2410 | sum |
| 88.5 | Percentage of pilot participants | ||
Group tests
For full view change to horizontal
| Number of all pulling test | 40 participants pulling |
32 participants pulling |
25 participants pulling |
16 participants pulling |
8 participants pulling |
Test group |
|---|---|---|---|---|---|---|
| 47 | 7 | 6 | 1 | 33 | Pre-school teachers, Bnei Brak | |
| 20 | 20 | Hevron yeshiva, Jerusalem | ||||
| 10 | 10 | Netiv HaDaat yeshiva, Jerusalem | ||||
| 11 | 1 | 10 | Mishckenot HaTora yeshiva, Bnei Brak | |||
| 18 | 18 | Rina Shel Tora yeshiva, Karmiel | ||||
| 106 | 1 | 47 | 6 | 19 | 33 | sum |
Participants
A total of 2410 people were sampled in the pilot, 2133 of them agreed to participate in the pilot (88.5%).
All the test tubes were divided into 5 groups:
- Bnei Brak kindergarten teachers – 8 samples per test tube – 33 test tubes.
- Yeshiva in Karmiel – 16 samples in each test tube – 19 girls.
- Bnei Brak kindergarten teachers – 25 samples in each test tube – 6 test tubes.
- Yeshivas in Jerusalem and Bnei Brak – 32 samples – per test tube, 47 test tubes.
- Seating in Bnei Brak – 40 samples – one test tube.
The sampling rate depended on the number of samples. The calculation was based on double sampling of 6 minutes per person.
Logistics
Registration
Registration of the subjects and their personal details: ID number, serial number of the test, test tube number, nozzle sampled for collection, nozzle sampled for individual test. Adhering personal stickers of all the members of the capsule on a document and attaching it to a test tube.Laboratory tests
- All samples were taken for testing to the MyHeritage lab.
- The MyHeritage laboratory transferred a sample from each of the collection tubes for further testing in the Central Virus Laboratory at Tel Hashomer Hospital.
- MyHeritage Lab kept all original samples until the end of the experiment.
- In order to validate the method, a comparison was made between all individual results and the collection results, and a comparison was made between the collection results of MyHeritage and the results of the Central Virus Laboratory in Tel Hashomer Hospital.
RESULTS AND CONCLUSIONS OF THE PILOT
Results – Pre-school teachers’ group:
All positive samples in the individual tests (15 of 654 who participated in the experiment, 2.2%) were identified in the group tests.
General sensitivity of the pooling method on this day – 100% (all 12 pools in which there was a positive sample were identified as such).
Specificity of the pooling method in this experiment – 100%. All 35 negative tests were identified as such.
Results - Yeshiva student groups:
Due to gaps in the registration, it was not possible to make a full comparison between the results of the individual tests and the aggregated tests.
The table shows a match in 41 out of 43 collections. 28 out of 29 positive reviews and 13 out of 14 positive reviews. When in most positive aggregates there were a number of positive samples.
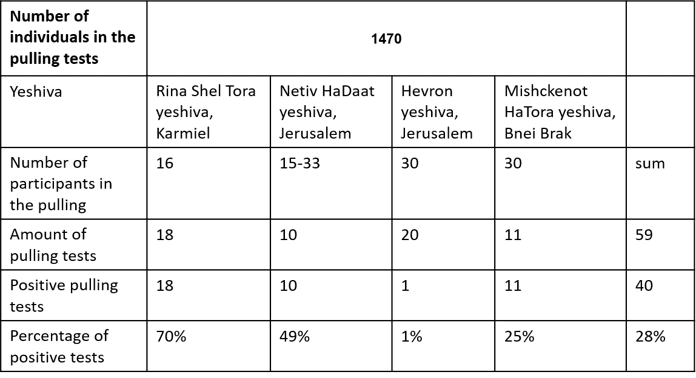
Conclusions:
The method is effective and enables the rapid identification of symptomatic and asymptomatic pre-patients in a group.
Swab pooling allows the identification of verified patients with a small impairment in the sensitivity of about 3 CT, resulting mainly from the sampling volume 5 times greater in the collection tubes.
Up to 40 swabs tested together, no masking problems were found, etc.
The processes must be automated throughout the supply chain from sampling to test result.
Continuation – urban/mass sampling in areas where the level of morbidity is high.